Learning Outcomes:
i. Comprehend the concept of hybridization and its role in determining molecular geometry.
ii. Explain the tetrahedral shape of alkanes based on sp3 hybridization.
iii. Describe the conformational isomers of ethane and their energy differences.
iv. Understand the ring strain in cycloalkanes and its relationship to molecular geometry.
v. Analyze the shapes of cyclopropane and cyclohexane using valence bond theory.
Introduction:
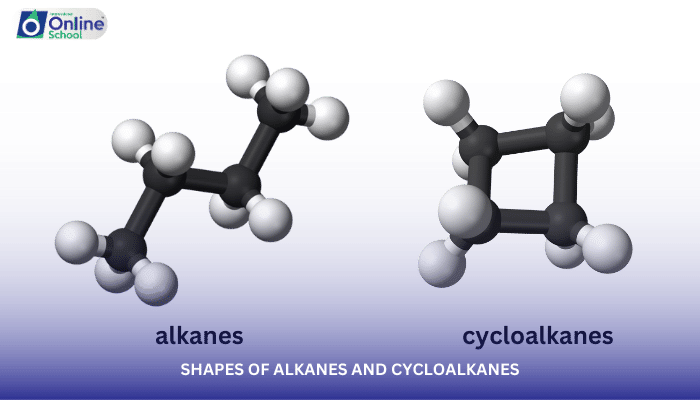
The shapes of organic molecules, particularly alkanes and cycloalkanes, play a crucial role in their physical and chemical properties. In this lesson, we will delve into the fascinating realm of molecular geometry, exploring the shapes of alkanes and cycloalkanes using the concept of hybridization and valence bond theory.
i. Hybridization: The Foundation of Molecular Geometry
Hybridization is a theoretical concept that describes the mixing of atomic orbitals to form new hybrid orbitals with a specific geometry. Understanding hybridization is essential for comprehending the shapes of organic molecules.
Sp3 Hybridization: In alkanes, carbon atoms undergo sp3 hybridization, where one s orbital and three p orbitals combine to form four hybrid orbitals arranged in a tetrahedral geometry.
ii. Tetrahedral Shape of Alkanes
Due to sp3 hybridization, carbon atoms in alkanes adopt a tetrahedral geometry, with bond angles of approximately 109.5 degrees. This tetrahedral shape is responsible for the overall structure of alkanes, including their open-chain and branched-chain forms.
iii. Conformational Isomers of Ethane
Ethane, the simplest alkane with two carbon atoms, exhibits two distinct conformational isomers: staggered and eclipsed.
Staggered Conformation: In the staggered conformation, the methyl groups (-CH3) are positioned far apart, minimizing steric repulsion and resulting in a lower energy state.
Eclipsed Conformation: In the eclipsed conformation, the methyl groups are positioned directly opposite each other, causing significant steric repulsion and leading to a higher energy state.
iv. Ring Strain in Cycloalkanes
Cycloalkanes, with their closed ring structures, introduce a unique challenge in molecular geometry. The rigid ring structure forces carbon atoms to deviate from their ideal tetrahedral angles, leading to ring strain.
Cyclopropane: Cyclopropane, a three-membered cycloalkane, experiences significant angle strain due to the severe deviation from tetrahedral angles. This strain contributes to its high reactivity.
Cyclohexane: Cyclohexane, a six-membered cycloalkane, adopts a chair conformation to minimize ring strain. The chair conformation allows carbon atoms to maintain angles close to 109.5 degrees, resulting in a lower energy state and increased stability.
The shapes of alkanes and cycloalkanes are determined by the interplay of hybridization and ring strain. Sp3 hybridization governs the tetrahedral geometry of alkanes, while ring strain influences the conformations of cycloalkanes. Understanding these concepts is crucial for predicting the physical and chemical properties of these organic compounds.